From Fermentation to Final Product: Small Molecule Manufacturing – a case study
White Paper CDMO October 2025
Biotechnology complements chemical synthesis in manufacturing of small organic molecules, often providing improved functionality and higher product purity. How does a biotechnology manufacturing process for small organics look like? What is the typical process flow and unit operations at large scale? Let us unpack a commercial campaign and highlight relevant trends, bioprocess metrics and challenges.
From Fermentation to Final Product: Small Molecule Manufacturing – a case study
Small molecules produced via microbial fermentation play a vital role in pharmaceutical, nutraceutical, and specialty chemical markets, representing a rapidly growing multibillion-dollar segment. While chemical synthesis remains dominant, biotechnology offers advantages such as desired molecule chirality, higher purity and sustainable production. This white paper provides a comprehensive overview of the manufacturing process for production of a small organic molecule, combining upstream (USP) and downstream processing (DSP), and shares operational insights from an industrial scale campaign.
Small organic molecules are low-molecular-weight compounds (<1 kDa) that play an important role as building blocks and regulators of biological processes. They include amino acids, sugars, organic acids, lipids, and fatty acids, and are distinct from macromolecules such as proteins, nucleic acids, and polysaccharides. Microbial fermentation has long been used to produce primary metabolites like amino acids, organic acids, and vitamins. Active pharmaceutical ingredients (APIs) and their intermediates are also small molecules, typically classified as secondary metabolites. Beyond well-known antibiotics, bio-manufactured drugs now target a wide range of (even non-infectious) diseases by interacting with specific biological targets. Advances in strain and metabolic engineering have significantly improved bioprocess productivity, enabling commercialization of additional secondary metabolites, often plant-derived, such as flavonoids for nutraceutical and personal care applications.
Process Overview
Any bioprocess starts with cultivation of either native or engineered microbial strain in stainless steel bioreactors with controlled conditions favorable for the product accumulation, followed by cell disruption and biomass debris removal (alternatively, the cell disruption step may be skipped in case of extracellular production), broth concentration and product purification to obtain the final product. Downstream processing and its employed unit operations largely depend on the nature and properties of the isolated molecule. Nevertheless, the steps illustrated in Figure 1 serve as an example of the manufacturing process for a water-soluble small molecule.
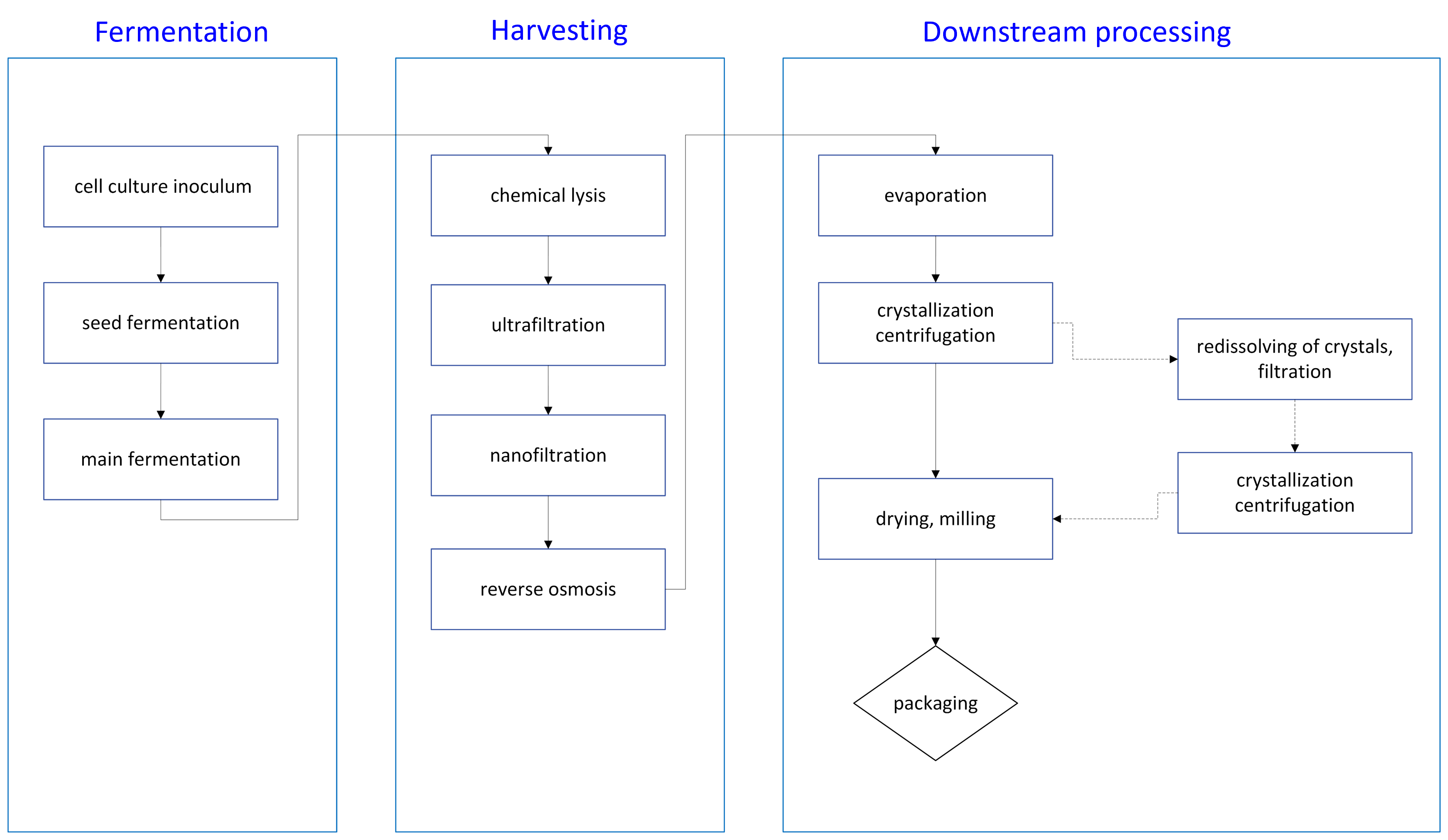
Figure 1: Block flow diagram of the manufacturing process.
Upstream processing
Upstream processing begins with inoculum preparation from a working cell bank (WCB). In this example, a genetically engineered bacterial strain was employed, leveraging metabolic engineering strategies such as targeted gene deletions, pathway overexpression, and cofactor balancing to enhance biosynthetic efficiency. The culture was expanded stepwise through a seed train, transferring from small inoculum volumes to progressively larger seed fermenters until reaching production scale. Main fermentation was operated in fed-batch mode, with feeding initiated upon depletion of initial carbon sources, indicated by a dissolved oxygen (DO) spike. The feeding strategy combined exponential feeding for rapid biomass growth with linear and later constant feeding to promote product formation. The production phase was triggered by a one-time temperature shift, otherwise temperature as well as pH were maintained constant, with pH controlled via automated ammonium hydroxide dosing. DO was regulated primarily through dynamic aeration, with only minor adjustments to agitation and pressure. Optimized feeding and DO control were critical to prevent accumulation of overflow metabolites such as acetate, which can significantly reduce process yield.
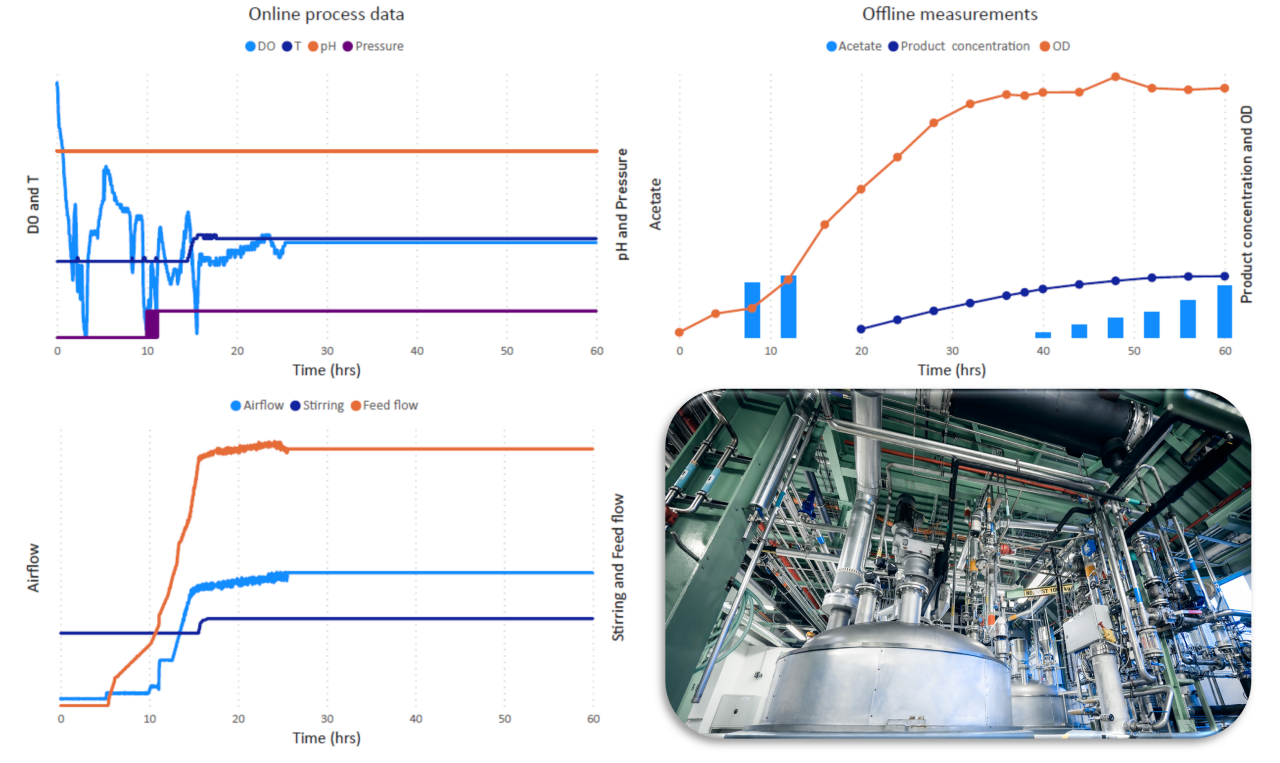
Figure 2: USP process profile. The charts illustrate the relative trends of bioprocess control parameters (dissolved oxygen - DO, temperature - T, pH, pressure, airflow, feed flow, stirring). On the right-hand side, product, biomass, and by-product (acetate) formation throughout the fermentation is displayed. Values and scale have been intentionally omitted to emphasize trend visualization and comparative patterns rather than precise figures. The picture displays the upper floor of the upstream manufacturing asset.
Harvesting and downstream processing (DSP)
After completion of the fermentation stage, the broth was subjected to cell lysis using a chemical agent. Cellular debris and high-molecular-weight impurities were removed through a series of filtration steps. The clarified broth was then concentrated via reverse osmosis followed by evaporation. The resulting concentrate underwent crystallization, and crystals were separated from the mother liquor by centrifugation. If the impurity profile did not meet specifications, the crystals were redissolved, filtered, and the crystallization–centrifugation cycle repeated. The process concluded with vacuum drying of the crystals and milling-sieving to achieve a uniform particle size in the final product (Figure 1).
Analytical control was maintained throughout the process, including in-process measurements, impurity profiling, and final product quality assessment using advanced instrumentation such as HPLC, FTIR, NIR, UV-VIS spectrophotometry, and moisture analyzers
Manufacturing performance and challenges
Even mature manufacturing processes can present operational challenges. During the showcased campaign, key issues included microbial contamination, oxygen transfer limitations leading to productivity deviations during fermentation, membrane fouling during harvesting, and extended drying times coupled with crystal handling difficulties in downstream processing (DSP).
These challenges were addressed through enhanced sterilization and cleaning protocols, optimized feeding strategies with dynamic feed control, adjustments to aeration and pressure, and equipment upgrades in case of dryer and advanced CIP (clean-in-place) systems for crystallizers.
The implemented improvements delivered an 18% increase in volumetric productivity during fermentation compared to the initial campaign stage. Overall process yield improved by 10% (Figure 3). A slight yield reduction in harvesting and DSP was observed, primarily due to stricter cleaning protocols introduced to ensure quality and maintain a success rate above 99% (Figure 3).
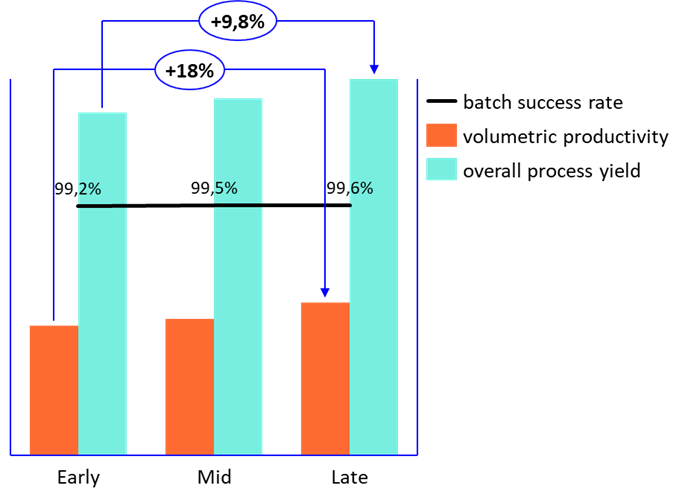
Figure 3: Manufacturing campaign performance. Values and scale have been intentionally omitted to emphasize trend visualization and percentual changes rather than precise figures.
Summary
Biotechnological production of small molecules offers a sustainable alternative to chemical synthesis, delivering high purity and stability. Optimized fed-batch fermentation, combined with robust downstream processing, can achieve high yields and consistent quality. Trade-offs between yield and quality are inevitable and should be managed proactively. Continuous improvement, through dynamic process control with real-time analytics, preventive maintenance, enhanced cleaning protocols, and equipment upgrades, is critical to remain competitive in a rapidly evolving market.
Our offer
- Over 40 years of experience with various bioprocesses
- Large as well as small molecule biomanufacturing
- Fully integrated CDMO services in the field of industrial biotechnology
- Engagement at any stage of product/process development
- 99% batch success rate
- Excellent on-time in full delivery performance with over 60 processes transferred to commercial scale in the past decade
- Strong focus on continuous process improvement
Authors information

Martin Venzhofer
Process Technologist

Vratislav Stovicek
Business Development and Project Execution Manager, CDMO
Acknowledgments
This work was funded by Arxada AG, Peter Merian-Strasse 80, 4052 Basel, Switzerland.
For further information and/or if you would like Arxada to support your project(s), get in touch with:
Arxada is an industry leader in science-based specialty chemicals that creates innovative chemistry and solutions. Comprised of two business units, Arxada’s Microbial Control Solutions (MCS) business provides more sustainable, science-based solutions that utilize differentiated capabilities in microbiology, actives delivery and formulation chemistry. Its manufacturing and unmatched regulatory expertise meets customer needs in variety of endmarkets, specifically, Professional Hygiene, Home & Personal Care, Paints & Coating, Wood Protection and Material Protection. Arxada’s Nutrition, Care & Environmental (NCE) business serves the needs of our partners in a diverse range of industries including food and feed supplements, aerospace, electronics, renewables, agriculture and industrial, as well as pharma intermediates. Leveraging our strong vertical integration into chemical building blocks, such as ethylene, acetylene, ketene/diketene and HCN, along with our fermentation capabilities and our deep technical expertise, NCE transforms customer needs into high performing solutions. This is achieved through direct product supply or contract development and manufacturing (CDMO). With major sites strategically located in the heart of Europe, Arxada secures its customers’ supply chains, while actively supporting their sustainability efforts.
Headquartered in Basel, Switzerland, the company’s global footprint spans 24 production sites and 14 R&D centers. Its 3,400 associates contribute daily to its overall success.
To learn more about Arxada, please visit: arxada.com and Arxada on LinkedIn
Disclaimer
All information in this presentation corresponds to Arxada’s knowledge on the subject at the date of publication, but Arxada makes no warranty as to its accuracy or completeness and Arxada assumes no obligation to update it. All information in this presentation is intended for use by recipients experienced and knowledgeable in the field, who are capable of and responsible for independently determining the suitability and to ensure their compliance with applicable law. Proper use of this information is the sole responsibility of the recipient. Republication of this information or related statements is prohibited. Information provided in this presentation by Arxada is not intended and should not be construed as a license to operate under or a recommendation to infringe any patent or other intellectual property right. All trademarks belong to Arxada or its affiliates or to their respective third parties and are used here only for informational purposes. Copyrighted material has been produced with permissions or under license, all other materials.
© 2025 Arxada Ltd.