From Hazard to Asset: The Role of Hydrogen Cyanide in Industrial Fine Chemical Synthesis
White Paper CDMO July 2025
Often perceived as a hazardous compound, hydrogen cyanide (HCN) is in fact an important industrial building block used in chemical synthesis across multiple industries. At Arxada, on-site HCN production is seamlessly integrated into a robust and flexible manufacturing network, ensuring controlled quality and supply continuity. Combined with deep expertise in HCN-based chemistry, it opens access to high-value intermediates. Furthermore, the agile CDMO (Contract Development and Manufacturing Organization) approach delivers tailor-made, end-to-end solutions that accelerate innovation and de-risk complex synthesis challenges.
From Hazard to Asset: The Role of Hydrogen Cyanide in Industrial Fine Chemical Synthesis
Hydrogen cyanide (HCN) is a reactive and versatile intermediate widely employed in the synthesis of nitriles, α-amino acids, and a range of fine and specialty chemicals. Owing to its dual nucleophilic and electrophilic character, HCN participates in several key transformations, including hydrocyanation of unsaturated compounds and the Strecker synthesis of α-aminonitriles. While its handling requires stringent safety controls due to its highly reactive and hazardous nature, HCN remains essential to numerous industrial-scale processes. At Arxada’s Visp facility, HCN is manufactured on-site through a fully integrated production network, allowing for stringent quality control, continuous availability, and cost efficiency. This infrastructure supports both large-scale manufacturing and tailored fine chemical synthesis, enabling the strategic and safe deployment of HCN across a broad range of applications.
HCN is a highly reactive and functionally diverse building block in industrial organic chemistry. Structurally simple, consisting of a hydrogen atom bonded to a carbon–nitrogen triple bond, HCN typically acts as a nucleophile via its conjugate base - the cyanide anion (CN⁻). This attribute enables its involvement in a broad range of key transformations, such as carbon–carbon bond-forming reactions. Although it requires stringent safety measures due to its hazardous nature, HCN continues to play a crucial role in production of nitriles, cyanohydrins, α-amino acids, and a wide range of fine and specialty chemicals, with global output exceeding one million tons annually. The use of HCN extends across multiple sectors, including polymer manufacturing, agrochemical synthesis, and pharmaceutical development. Large proportion of produced HCN is used in hydrocyanation processes. HCN adds across unsaturated carbon–carbon bonds to form aliphatic nitriles, which can easily undergo further transformations. E.g. adiponitrile serves as a precursor to Nylon-6,61 with application in automotive and textile industry, acetone cyanohydrin2 is primarily used in synthesis of methyl methacrylate, a key component of acrylic polymers. In the context of nitrogen-containing building blocks, the Strecker synthesis employs HCN to form α-aminonitriles via addition to imines derived from aldehydes or ketones and ammonia.3 Moreover, HCN can be applied in Sandmeyer-type cyanation reactions, where aryl diazonium salts are converted into aryl nitriles using CuCN.4
At Arxada’s Visp facility, HCN is produced on-site as part of a fully integrated production infrastructure. The Visp’s cracker (Acetylene Generating Unit, AGU) produces (among other streams) methane that is fed into the cyanide unit, where it is subsequently converted into HCN (Figure 1). This level of backward integration provides Arxada with full control over production parameters, including purity, throughput, and supply continuity, critical for both high-volume and precision fine chemical applications. Based on the technologies derived thereof, Arxada can offer a variety of basic chemicals and performance intermediates as portfolio products as well as CDMO tailor-made solutions.
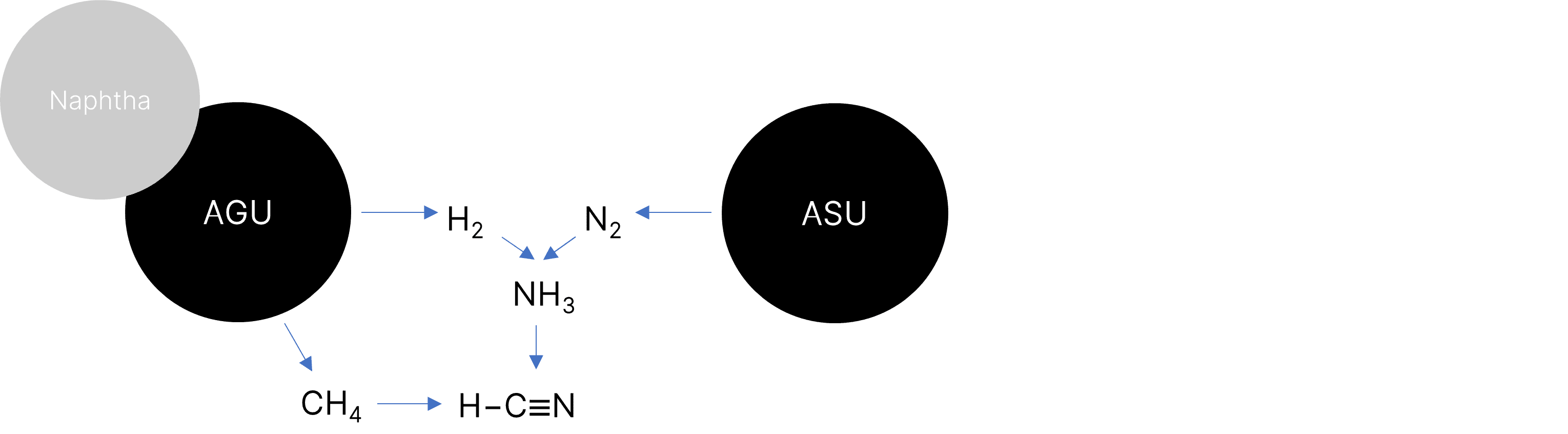
Figure 1: HCN backward integration at Arxada (Visp, Switzerland). AGU – acetylene generation unit, ASU – air separation unit.
Selected Applications of HCN in Synthetic Chemistry:
1. Strecker Synthesis – Formation of α-Amino Acids
The Strecker synthesis remains a robust route for the formation of α-amino acids or α-amino alcohols via the condensation of an aldehyde or ketone with ammonia, followed by nucleophilic addition of HCN to form an α-aminonitrile. Acidic hydrolysis yields the corresponding α-amino acid. This method, first described by Adolph Strecker in 1850, has been adapted for both batch and continuous flow operations and is still industrially employed particularly in methionine production5 for animal feed, or production of selected non-proteinogenic amino acids. These can be further be employed in the synthesis of various pharmaceuticals.6 Additionally, α-aminonitriles are precursors to chiral amines, which are often found in active ingredients of pharmaceuticals and agrochemicals.7

2. Cyanohydrin Formation – Addition to Carbonyl Compounds
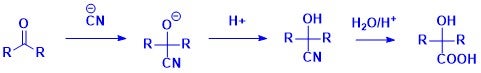
HCN readily undergoes nucleophilic addition to aldehydes and ketones to afford cyanohydrins, which contain both hydroxyl and nitrile functional groups. Chiral cyanohydrins are valuable intermediates in asymmetric synthesis, and their hydrolysis leads to α-hydroxy acids. Industrial-scale applications include synthesis of key building blocks in the synthesis of cardiovascular drugs, antibiotics, and other APIs (active pharmaceutical ingredients).8 In agrochemicals, cyanohydrins derived from various aldehydes are used to produce herbicides and insecticides.9 Additionally, hydrolysis of cyanohydrin intermediates yields lactic acid derivatives, which find applications in biodegradable plastics, cosmetics, and food additives. 10,11
3. Sandmeyer-Type Cyanation – Synthesis of Aromatic Nitriles
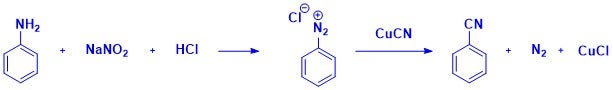
The Sandmeyer reaction enables the transformation of aromatic amines into substituted aromatic compounds via diazonium salt intermediates. In the cyanation variant, copper(I) cyanide is employed to install nitrile groups onto aromatic rings.12 This transformation is widely used in the production of benzonitrile derivatives, which are essential in the synthesis of some APIs. In the agrochemical industry, nitrile-containing herbicides are synthesized via Sandmeyer cyanation of halogenated phenols.13 The dye and pigment industry also relies on this reaction to produce azo dyes, where aromatic nitriles act as versatile precursors for further functionalization.14
Summary
This white paper outlines the key chemical transformations and industrial applications enabled by hydrogen cyanide (HCN). Despite its hazardous nature, HCN remains a cornerstone of chemical synthesis across multiple sectors. Arxada is uniquely positioned to harness HCN safely, supporting both large-scale industrial processes and customized client-specific projects. Through its vertically integrated production model and advanced chemical development capabilities, we can effectively address common challenges such as production costs, safety measures, and overcome logistical constraints related to storage and transportation.
Our offer
- Backward integrated cyanide operations
- Portfolio of HCN-derived intermediates
- Experience with various chemistries employing HCN
- Fully integrated CDMO services and tailor-made solutions
- Focus on what matters to you
Authors information

Ana Maria Montagut
Technical Evaluation Manager CDMO

Vratislav Stovicek
Business Development Analyst CDMO
Acknowledgments
This work was funded by Arxada AG, Peter Merian-Strasse 80, 4052 Basel, Switzerland.
References
1) Blanco, D. E.; Dookhith, A. Z.; Modestino, M. A. Enhancing Selectivity and Efficiency in the Electrochemical Synthesis of Adiponitrile. React. Chem. Eng. 2018, 4 (1), 8–16. https://doi.org/10.1039/C8RE00262B.
(2) Bartsch, M.; Baumann, R.; Haderlein, G.; Flores, M. A.; Jungkamp, T.; Luyken, H.; Scheidel, J.; Siegel, W. Method for the Production of Adipodinitrile by Hydrocyanation of 1,3-Butadiene. US20080221351A1, September 11, 2008. https://patents.google.com/patent/US20080221351/en.
(3) Ashenhurst, J. The Strecker Synthesis of Amino Acids. Master Organic Chemistry. https://www.masterorganicchemistry.com/2018/11/12/the-strecker-synthesis-of-amino-acids.
(4) Akhtar, R.; Zahoor, A. F.; Rasool, N.; Ahmad, M.; Ali, K. G. Recent Trends in the Chemistry of Sandmeyer Reaction: A Review. Mol. Divers. 2022, 26 (3), 1837–1873. https://doi.org/10.1007/s11030-021-10295-3.
(5) Geiger, F. D.; Halsberghe, B.; Hasselbach, H.-J. D.; Hentschel, K. D.; Huthmacher, K. D.; Körfer, M.; Mannsfeld, S.-P. D.; Tanner, H. D.; Theissen, F. D.; Vanrobaeys, J.; Willigerodt, K. D. Process for the Preparation of D,L-Methionine or Salts Thereof. EP0780370A2, June 25, 1997. https://patents.google.com/patent/EP0780370A2/en.
(6) Masamba, W. Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis. Molecules 2021, 26 (6), 1707. https://doi.org/10.3390/molecules26061707.
(7) Ullah, B.; Gupta, N. K.; Ke, Q.; Ullah, N.; Cai, X.; Liu, D. Organocatalytic Synthesis of α-Aminonitriles: A Review. Catalysts 2022, 12 (10), 1149. https://doi.org/10.3390/catal12101149.
(8) Ishida, H.; Hirota, M. Process for the Production of a Mandelonitrile Compound. EP2311800B1, September 19, 2018. https://patents.google.com/patent/EP2311800B1/es.
(9) Fujiwara, N.; Nakagawa, K.; Kinoshita, Y.; Midorikawa, K. Process for Production of Cyanohydrin Compound, and Process for Production of Alpha-Hydroxyester Compound. EP2213656B1, January 18, 2017. https://patents.google.com/patent/EP2213656B1/en.
(10) Gregory, R. J. H. Cyanohydrins in Nature and the Laboratory: Biology, Preparations, and Synthetic Applications. Chem. Rev. 1999, 99 (12), 3649–3682. https://doi.org/10.1021/cr9902906.
(11) Kim, J.; Kim, Y.-M.; Lebaka, V. R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8 (11), 609. https://doi.org/10.3390/fermentation8110609.
(12) Moody, D. J.; Hamill, N. A. Process for the Preparation of Aryl Diazonium Salts and Reaction with Nucleophiles. EP1636150A1, March 22, 2006. https://patents.google.com/patent/EP1636150A1/ja.
(13) Powell, G. S.; Sinodis, D. N.; Timmons, P. R.; Wu, T.-T. Pesticidal 1-Arylimidazoles. EP0396427B1, September 13, 1995. https://patents.google.com/patent/EP0396427B1/pl.
(14) Mo, F.; Qiu, D.; Zhang, L.; Wang, J. Recent Development of Aryl Diazonium Chemistry for the Derivatization of Aromatic Compounds. Chem. Rev. 2021, 121 (10), 5741–5829. https://doi.org/10.1021/acs.chemrev.0c01030.
For further information and/or if you would like Arxada to support your project(s), get in touch with:
Arxada is an industry leader in science-based specialty chemicals that creates innovative chemistry and solutions. Comprised of two business units, Arxada’s Microbial Control Solutions (MCS) business provides more sustainable, science-based solutions that utilize differentiated capabilities in microbiology, actives delivery and formulation chemistry. Its manufacturing and unmatched regulatory expertise meets customer needs in variety of endmarkets, specifically, Professional Hygiene, Home & Personal Care, Paints & Coating, Wood Protection and Material Protection. Arxada’s Nutrition, Care & Environmental (NCE) business serves the needs of our partners in a diverse range of industries including food and feed supplements, aerospace, electronics, renewables, agriculture and industrial, as well as pharma intermediates. Leveraging our strong vertical integration into chemical building blocks, such as ethylene, acetylene, ketene/diketene and HCN, along with our fermentation capabilities and our deep technical expertise, NCE transforms customer needs into high performing solutions. This is achieved through direct product supply or contract development and manufacturing (CDMO). With major sites strategically located in the heart of Europe, Arxada secures its customers’ supply chains, while actively supporting their sustainability efforts.
Headquartered in Basel, Switzerland, the company’s global footprint spans 24 production sites and 14 R&D centers. Its 3,400 associates contribute daily to its overall success.
To learn more about Arxada, please visit: arxada.com and Arxada on LinkedIn
Disclaimer
All information in this presentation corresponds to Arxada’s knowledge on the subject at the date of publication, but Arxada makes no warranty as to its accuracy or completeness and Arxada assumes no obligation to update it. All information in this presentation is intended for use by recipients experienced and knowledgeable in the field, who are capable of and responsible for independently determining the suitability and to ensure their compliance with applicable law. Proper use of this information is the sole responsibility of the recipient. Republication of this information or related statements is prohibited. Information provided in this presentation by Arxada is not intended and should not be construed as a license to operate under or a recommendation to infringe any patent or other intellectual property right. All trademarks belong to Arxada or its affiliates or to their respective third parties and are used here only for informational purposes. Copyrighted material has been produced with permissions or under license, all other materials.
© 2025 Arxada Ltd.