From process to product: Drying as the final transformative step in industrial biotechnology
White Paper CDMO September 2025
How does industrial biotechnology manufacturing ensure product stability, scalability, and shelf life in the final stage of production? What technologies best preserve sensitive biomolecules without compromising quality? Let us explore final steps in bioproduct manufacturing, where technologies such as spray drying, freeze drying, and vacuum drying are employed based on product requirements and properties.
From process to product: Drying as the final transformative step in industrial biotechnology
Drying is a fundamental unit operation in industrial biotechnology, essential for converting liquid, semi-liquid, or wet biological products into stable, dry solids. The primary objectives of drying are to ensure product stability, extend shelf life, facilitate transport, and minimize the risk of microbial contamination or product degradation. The selection of an appropriate drying method is a complex decision, influenced by the physicochemical properties of the product, its sensitivity to temperature and oxygen, and the economic and environmental requirements of the process. This paper provides a comprehensive overview of the main drying technologies used in industrial biotechnology and Arxada CDMO’s (Contract Development and Manufacturing Organization) capabilities and expertise.
In industrial biotechnology, the journey from fermentation to final product involves a series of downstream processing (DSP) steps designed to purify, stabilize, and prepare the product for shipment. The final phase of this journey, often referred to as product polishing, encompasses the operations that transform a biologically active substance into a form that is stable, transportable, and convenient for end use. A typical operation employed in the product polishing stage is drying. Drying is defined as the removal of water or other solvents from a material by evaporation. The driving force for drying is the difference in vapor pressure between the wet material and the surrounding air or vacuum. The rate of drying depends on several factors, including product properties (particle size, shape, porosity, composition), process parameters (temperature, humidity, air flow, pressure), and water activity. The objective is to reduce water activity to a level that ensures stability, chemical integrity, and longer shelf life. Biotechnological products, such as enzymes, proteins, other biopolymers (e.g. polysaccharides), active biomass, and specialty chemicals are often highly sensitive to heat and oxidation. Water in bioproducts exists as free water (extracellular, intracellular), its removal is relatively easy and essential for reduction of bulk moisture, and bound water (within cell structures), which is more challenging to extract, and its removal largely impacts product stability and activity. Therefore, the appropriate drying method must be selected and process parameters fine-tuned to achieve desired product quality and efficient water removal, considering product characteristics, type of water to be removed and economic requirements of the process. The most common types of drying technologies used in biotechnology include spray drying, freeze drying (lyophilization), and vacuum drying, each with specific advantages and limitations (Table 1).
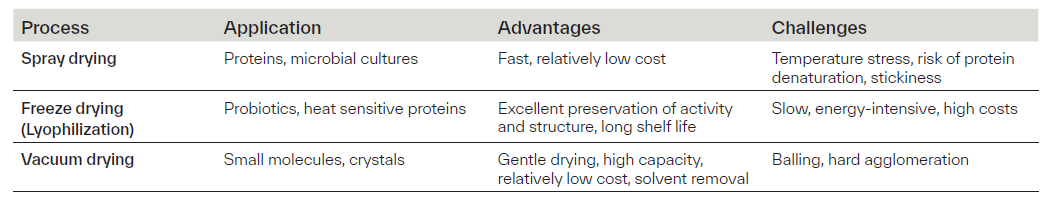
Table 1. Overview of the most common types of drying technologies in industrial biotechnology
Spray drying is a widely adopted technique in industrial biotechnology for converting liquids into dry powders. The process involves atomizing the liquid into fine droplets, which are rapidly dried by a stream of hot air in a controlled chamber. This results in powders with tailored particle size, density, surface area, and crystallinity, ideal for applications involving various proteins, microbial cultures, and certain specialty chemicals. Its advantages include continuous operation, scalability, and cost-efficiency. However, thermal stress remains a key limitation, particularly for heat-sensitive biomolecules such as enzymes and active biomass. To mitigate this, protective carriers or encapsulation strategies may be employed. Additionally, challenges like stickiness and wall deposits, especially in sugar- or protein-rich formulations, require careful process design.
Arxada CDMO possesses spray drying capabilities across all relevant scales, from lab-scale development to full-scale production (Table 2). By actively controlling critical parameters such as inlet/outlet air temperatures, atomization type and pressure, feed rate, and airflow, we ensure precise management of drying kinetics and material behavior. This enables consistent product quality, minimized degradation, and scalable performance tailored to the needs of various products in the food or feed industry.
Freeze drying, or lyophilization, is the preferred method for stabilizing high-value, thermally sensitive bioproducts such as enzymes and viable probiotics. The process consists of three key stages: freezing the material, primary drying via sublimation under vacuum, and secondary drying to remove residual moisture. This technique excels at preserving biological activity, structural integrity, and rehydration performance. Its effectiveness in removing both free and bound water makes it especially suitable for delicate formulations, where product stability is prioritized over cost as freeze drying is energy-intensive and time-consuming.
At Arxada, freeze drying can be optimized through precise control of critical parameters such as shelf temperature, which governs sublimation and desorption rates; chamber pressure, which maintains the vacuum needed for phase change; product temperature to avoid product structural damage; and freezing rate that influences ice crystal formation, which affects drying efficiency and final product texture.
Vacuum drying is another gentle and effective method for processing heat- and oxygen-sensitive bioproducts, such as wet crystals or materials containing flammable solvents. By lowering the ambient pressure, the boiling point of water is reduced, allowing moisture to be removed at significantly lower temperatures, minimizing thermal degradation and preserving product integrity. This technique is particularly useful for removing bound water without exposing the product to harsh conditions. However, it is typically a batch process and may present challenges such as particle agglomeration or mechanical instability. Arxada is equipped with various drying equipment at production scale (Table 2), allowing for controlling parameters such as vacuum level, drying temperature, agitator speed, and product load. These factors influence heat transfer efficiency, drying uniformity, and mechanical stability of the final product.
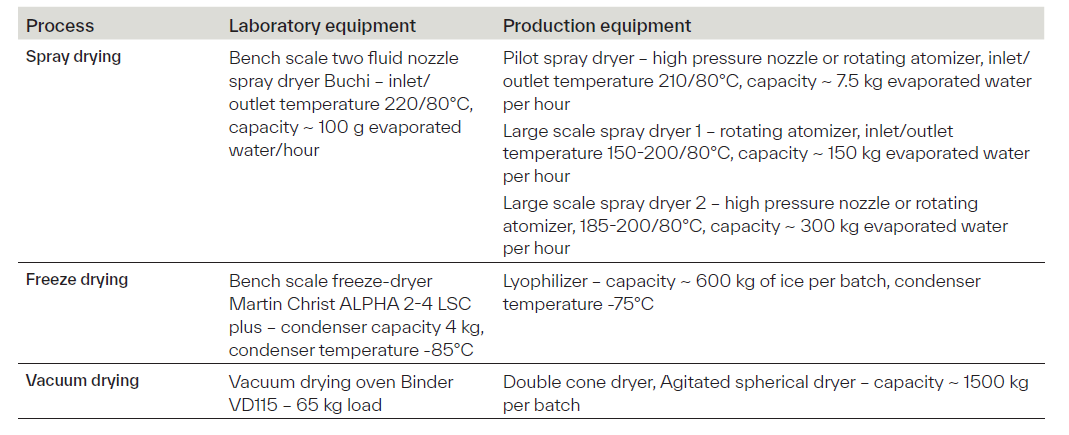
Table 2. Arxada’s drying capabilities across scales.
Summary
This white paper explores the most common drying technologies in industrial biotechnology, essential for converting often delicate biological substances into stable and transportable products. Each method is discussed in terms of its principles, applications, advantages, and challenges. We highlight the importance of selecting appropriate drying technology based on product characteristics, its intended end application, and economics. Arxada’s capabilities across lab, pilot, and production scales are showcased. By tailoring drying solutions to specific biotechnological applications, Arxada CDMO ensures high product quality, stability, and scalability, supported by a strong track record in process transfer and commercial manufacturing.
Our offer
- Fully integrated CDMO services in the field of industrial biotechnology
- Engagement at any stage of product/process development
- From product generation to finish – various drying capabilities in laboratory, pilot and production scale
- 99% batch success rate
- Excellent on-time in full delivery performance with over 60 processes transferred to commercial scale in the past decade
- Strong focus on continuous process improvement
Authors information

Pavel Havelka
Evaluation Manager

Vratislav Stovicek
Business Development and Project Execution Manager, CDMO
Acknowledgments
This work was funded by Arxada AG, Peter Merian-Strasse 80, 4052 Basel, Switzerland.
For further information and/or if you would like Arxada to support your project(s), get in touch with:
Arxada is an industry leader in science-based specialty chemicals that creates innovative chemistry and solutions. Comprised of two business units, Arxada’s Microbial Control Solutions (MCS) business provides more sustainable, science-based solutions that utilize differentiated capabilities in microbiology, actives delivery and formulation chemistry. Its manufacturing and unmatched regulatory expertise meets customer needs in variety of endmarkets, specifically, Professional Hygiene, Home & Personal Care, Paints & Coating, Wood Protection and Material Protection. Arxada’s Nutrition, Care & Environmental (NCE) business serves the needs of our partners in a diverse range of industries including food and feed supplements, aerospace, electronics, renewables, agriculture and industrial, as well as pharma intermediates. Leveraging our strong vertical integration into chemical building blocks, such as ethylene, acetylene, ketene/diketene and HCN, along with our fermentation capabilities and our deep technical expertise, NCE transforms customer needs into high performing solutions. This is achieved through direct product supply or contract development and manufacturing (CDMO). With major sites strategically located in the heart of Europe, Arxada secures its customers’ supply chains, while actively supporting their sustainability efforts.
Headquartered in Basel, Switzerland, the company’s global footprint spans 24 production sites and 14 R&D centers. Its 3,400 associates contribute daily to its overall success.
To learn more about Arxada, please visit: arxada.com and Arxada on LinkedIn
Disclaimer
All information in this presentation corresponds to Arxada’s knowledge on the subject at the date of publication, but Arxada makes no warranty as to its accuracy or completeness and Arxada assumes no obligation to update it. All information in this presentation is intended for use by recipients experienced and knowledgeable in the field, who are capable of and responsible for independently determining the suitability and to ensure their compliance with applicable law. Proper use of this information is the sole responsibility of the recipient. Republication of this information or related statements is prohibited. Information provided in this presentation by Arxada is not intended and should not be construed as a license to operate under or a recommendation to infringe any patent or other intellectual property right. All trademarks belong to Arxada or its affiliates or to their respective third parties and are used here only for informational purposes. Copyrighted material has been produced with permissions or under license, all other materials.
© 2025 Arxada Ltd.